
Sublimation is the process of changing the material from its solid to gaseous form without it being liquid, according to Physics.ĭry ice can be created by blending and freezing carbon dioxide (CO 2 ). In this process, first, the steel is converted into the molten state by heating at a high temperature and then poured into a mould for any desirable shape. Melting of steel is done to make different types of products of steel. An impure solid often melts across a range of temperatures below the melting point of the primary component, whereas this process happens in pure crystalline solids at a set temperature known as the melting point. Melting occurs when heat is applied, and a solid transforms into a liquid. When a liquid's molecules slow down enough to attract one another into permanent positions as a solid, this process is known as freezing.įreezing is used in food preservation techniques that involve decreasing the temperature to stop bacterial development. The process through which a material transforms from a liquid to a solid is known as freezing. The heat escaping from the cup aids in the cooling of the coffee. The steam that rises from a hot cup of coffee is a typical illustration of evaporation. Evaporation merely refers to a form of vaporisation that mostly occurs below the boiling point of water.

Boiling is the term for the evaporation process.
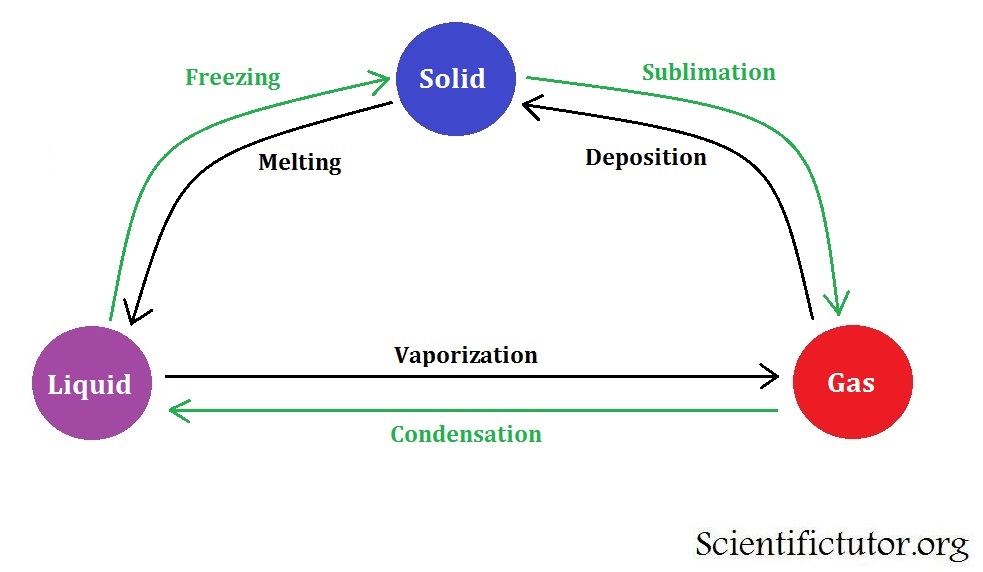
Vaporisation is the process by which a material is transformed from its liquid or solid state into its gaseous (vapour) state. These droplets condense onto microscopic airborne dust particles to form clouds. Tiny water droplets form when the temperature of the water vapour reaches the dew point or lower. When the gas is converted into liquid form by freezing or by any other method, then it is known as the condensation process.Īs the water vapour cools in the sky, clouds are created. Gas to solid process can be in a carbon dioxide fire extinguisher is initially filled with gaseous carbon dioxide, but the increased pressure inside the canister causes this to solidify and get expelled as a white powder when extinguishing a fire.Ĭondensation and Vaporisation Condensation Using colder temperatures and higher pressure, the gas particles skip the liquid phase and settle into a solid to create dry ice. To create dry ice, gaseous carbon dioxide must first be drawn out of the atmosphere. The reason it is termed deposition is that gaseous particles are deposited into the solid form. Particles in a solid have less kinetic energy and vibrate more slowly without changing position. Particles in a gas have a greater quantity of kinetic or moving energy, and they vibrate at a high rate. Thermal energy must be removed for a material to transition from its physical state from gas to solid this process is called deposition. When the ice melts, the container contains liquid and solid water both. This usually occurs during the change from one phase to another.

Two phases may dwell in the same container at the same time. The intermolecular forces acting on the material's molecules and atoms significantly impact the temperature and pressure at which the substance will change.

Every substance is in one of these three phases at a certain temperature. Each material can transform into three phases: solid, liquid, or gas.


 0 kommentar(er)
0 kommentar(er)
